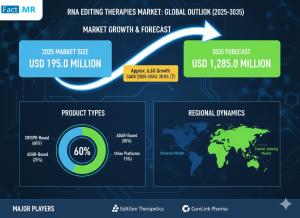
RNA Editing Therapies Market Valued USD 195.0 million in 2025 | Led by Shape Therapeutics, Korro Bio, ProQR Therapeutics
The global RNA editing therapies market is projected to grow from USD 195.0 million in 2025 to USD 1,285.0 million by 2035, advancing at a CAGR of 20.8%
ROCKVILLE, MD, UNITED STATES, October 10, 2025 /EINPresswire.com/ -- According to recent market studies, the RNA Editing Therapies Market is expected to grow significantly from 2025 to 2034. One forecast estimates a market size of about USD 15.95 billion in 2025, rising to approximately USD 27.01 billion by 2034, at a compound annual growth rate (CAGR) of about 6.0%. These figures reflect rising investment, improving delivery technologies, and expanding therapeutic applications. What was once primarily laboratory innovation is now becoming a robust area of therapeutic development.Key Drivers & Technological Trends:
Several factors are converging to drive the maturation of RNA editing therapies:
Therapeutic Precision & Safety: RNA editing allows for modulation or correction of disease-relevant RNA transcripts without permanent modification of the genome. This appeals especially for rare or early-onset genetic disorders, where DNA editing may pose higher risk.
Advances in Delivery Platforms: Lipid nanoparticles (LNPs), conjugated oligos, and enhanced viral or non-viral vectors are gaining prominence—especially for systemic or central nervous system (CNS) delivery. As delivery technology improves, therapeutic reach broadens.
Diverse Editing Modalities: ADAR-based (adenosine deaminase acting on RNA) editing is currently dominant, especially for base editing (A-to-I / G) with lower immunogenicity. Other modalities like exon editing are rapidly gaining traction, particularly as researchers work toward durable, more permanent but still reversible therapies.
Strong R&D & Regulatory Support: The combination of academic innovation, biotech startups, and larger pharma investments is fueling accelerated preclinical and early clinical work. Regulatory agencies are increasingly open to pathways for RNA editing, especially for rare disease indications.
Regional Insights: United States & Europe:
United States:
The U.S. stands out as the current leader in the RNA editing therapies market. Its strength lies in a well-established biotech ecosystem, large pools of venture funding, top-tier research institutions, and early regulators comfortable with innovation. Many of the high-profile players in the RNA editing space—both startups and established pharma companies—are U.S.-based or have strong U.S. presences. Clinical trials are more likely to launch here first, especially in genetic liver diseases, ophthalmologic disorders, and neurological rare diseases. On the regulatory side, the U.S. FDA’s willingness to engage with novel editing modalities has helped shorten development timelines.
Europe:
Europe is catching up quickly. Research hubs in Germany, the UK, France, and the Nordic countries are increasingly active in RNA editing science. European regulatory frameworks, while thorough, often offer strong incentives for treatments of rare diseases and have growing pathways for approvals of novel modalities. European academic–industry collaborations are contributing to innovation, particularly in delivery methods and precision editing. Additionally, patient advocacy and orphan disease designations help shape markets for RNA editing therapies in Europe. However, pricing, reimbursement, and adoption timelines tend to be more conservative compared to the U.S., due to cost containment and stricter assessment of clinical evidence.
Request for Discount: https://www.factmr.com/connectus/sample?flag=S&rep_id=11179
Segments, Indications & Competitive Landscape
Breaking the market into segments reveals where opportunity and competition are concentrated:
Editing Modality / Mechanism:
ADAR-mediated base editing is currently the largest share holder, particularly for A-to-I corrections. Exon editing is expected to grow fastest, especially for CNS and multi-organ rare disorders.
Therapeutic Intent:
“Repeat-dosed corrective editing” dominates now, used in disorders where ongoing dosing is feasible and safer. Moving forward, “one-time durable editing” therapies are expected to command attention, particularly in gene correction strategies where long-lasting effect is valuable.
Delivery Platforms:
LNPs and conjugated oligonucleotide systems are key for liver or systemic targets. But improved viral vectors (AAV serotypes) and non-viral delivery focused on CNS, ocular, or local administration are emerging as fast growth areas.
Indications / Disease Areas:
Rare genetic liver and ocular disorders have led market value so far, leveraging well-understood biology and relatively easier delivery. Neurological, multi-organ, or CNS-targeted rare genetic disorders are forecasted to grow fastest as delivery technologies and safety data improve.
Key Players:
Major organizations shaping the space include Ascidian Therapeutics, Beam Therapeutics, Korro Bio, Moderna, ProQR Therapeutics, Shape Therapeutics, Roche, Wave Life Sciences, among others. Many of them are working on different modalities, delivery platforms, or disease indications.
Recent Developments & Innovation Signals:
In June 2024, Roche partnered with Ascidian Therapeutics to license RNA exon editing technology focused on neurological diseases. This reflects growing commercial interest in durable RNA editing approaches.
Clinical proof-of-concept data from companies like Wave Life Sciences and Korro Bio (e.g., in alpha-1 antitrypsin deficiency) are attracting investor interest and confidence in RNA editing platforms.
Improvements in molecular tools (e.g., higher fidelity editors, reduced off-target effects) and better delivery strategies (engineered LNPs, improved vector tropism) are enhancing safety and efficacy profiles—key for regulatory approvals.
Buy Now at USD 2900: https://www.factmr.com/checkout/11179
Challenges & Restraints:
While promising, the RNA editing therapies market still must navigate several hurdles:
Delivery to Hard-to-Reach Tissues: The CNS, retina, or certain deep organs remain challenging; crossing barriers (e.g., blood–brain barrier) safely and efficiently is complex.
Safety and Off-Target Risks: RNA editing must avoid undesirable immune responses, off-target edits, or unintended editing of non-target transcripts. Long-term safety data are still limited.
Regulatory Pathways & Reimbursement: Novel modalities often require new regulatory frameworks, and payers may be cautious unless clinical and health-economic evidence is strong.
Manufacturing, Scalability & Cost: High costs of synthesis, delivery manufacturing (especially for LNPs or specialized vectors), QC, and challenges in scaling remain major considerations.
Check out More Related Studies Published by Fact.MR Research:
RNA Transcriptome Profiling Test Market is valued at $7.92B in 2023, is projected to grow at a 14.5% CAGR
RNA-based Therapeutics and Vaccine Market is projected to grow from US$ 180.5 million in 2024 to US$ 1.24 billion by 2034, with a CAGR of 21.3%.
S. N. Jha
Fact.MR
+1 628-251-1583
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.